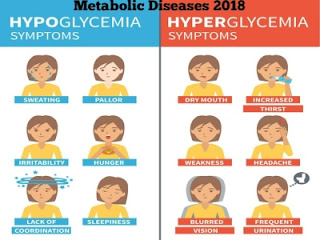
At what time diabetes patients can drink milk?

A change in breakfast schedule may give benefits for the management of type 2 diabetes . A study inspected the impacts of devouring high-protein milk at breakfast on blood glucose levels and satiety after breakfast and after a moment dinner. Milk devoured with breakfast cereal diminished postprandial blood glucose concentration compared with water, and high dairy protein concentration decreased postprandial blood glucose concentration compared with ordinary dairy protein concentration. The high-protein treatment moreover diminished appetite after the lunch compared with the low-protein proportionate. Metabolic diseases are on the rise universally, with type 2 diabetes and weight as driving concerns in human well-being. In this way, there's impulse to create dietary procedures for the risk reduction and management of obesity and diabetes to engage buyers to progress their individual well-being. In this randomized, controlled, double-blinded study, the analysts in